Laboratory Informatics – the Devil is in the Details
What is a LIMS?
A LIMS is a lot of things to a lot of people. Ask 10 people in the industry and expect 10 different answers. Let’s first begin with what the acronym stands for.
LIMS = Laboratory Information Management System
And next, let’s align on a definition of what a LIMS is.
DEFINITION: As paraphrased from Wikipedia, a software-based solution that supports laboratory operations. Key features include workflow and data tracking support, flexible architecture, and data exchange interfaces, often finding utility in regulated environments. Features and uses of LIMS have evolved over the years from simple sample tracking to an enterprise resource planning tool that manages multiple aspects of laboratory informatics.
There are two terms within the definition that perhaps need to be further explained before the details of a LIMS can be expanded: workflow and data tracking. When lab scientists read or hear the word workflow, here are just a few of the assumptions they make:
- Wet lab operations of a process, such as an extraction workflow.
- The entire workflow a sample follows through the lab from sample management to wet lab operations to analysis and ultimately to reported result(s).
- The movement of data within a system, such as with an ERP system and the generation of requisitions for items, orders placed, receipt of supplies, inventory received.
When lab scientists consider data tracking, there may be a few assumptions made, such as:
- It’s referring to the barcode on a sample tube.
- Representing a sample identifier in a sample management system.
- It can be all encompassing and address tracking of samples to verified results for those samples.
The main point is that data tracking is a primary function of a LIMS and aligning on the critical data that needs to be tracked is one of the first steps in scoping the connectivity of a LIMS.
By now, most readers are wondering whether a LIMS is even necessary and often, it’s the most common question that needs to be addressed from the onset. A few basic questions to consider:
- Are you able to manage the tracking of samples using a spreadsheet?
- Will you be able to continue to track your samples using a spreadsheet in the coming 12 months, or will your sample volumes grow beyond the capabilities of Excel or the technician doing data entry using Excel?
- Are you tracking everything you need to track or are you abbreviating what’s being tracked because the data entry aspects are too cumbersome?
Are you confident your process is being followed and your results are verifiable? - Can you judge the health of your lab or lab processes from your spreadsheets?
- If required, would your lab processes and spreadsheets withstand the scrutiny of an auditing authority?
Not every LIMS solution is created equally. The most common misconception about a LIMS is that laboratories hope to buy an off-the-shelf solution that can easily be deployed in their lab. Often, LIMS vendors come in and claim that their LIMS offers pre-canned workflows that will work exactly according to your needs. Do you think your lab does something exactly the same as every other lab? In the 20+ years this author has been supporting global laboratories, it’s rare to find a lab that is mirroring every facet of their operations to another lab. For that reason, customization in the LIMS world is not a dirty word; it’s critical to the successful implementation of each lab’s individualized SOPs.
Another fallacy to address about a LIMS is that it’s a static system. When deploying a LIMS system, a laboratory often notices areas of improvement because if implemented correctly, bottlenecks within the lab can be highlighted. One example could be where a higher than average failure rate on a particular reagent is tracked and as such, the QC metrics are investigated and require adjustments on incoming QC and quarantine of parts received. In addition, a LIMS system needs to evolve to address expanding lab needs. An example could be for a laboratory that introduces a new test and all required components of the new test would be added to the LIMS as part of the regulatory requirements of the lab. A LIMS is not a static system, but more appropriately a dynamic tool to help keep the laboratory efficient, compliant and organized.
Earlier, the ideas around workflow were stated. It’s important to understand that some LIMS solutions focus on certain areas of lab operations, mainly the wet lab environment. By this we mean that a LIMS tracks a sample by its assigned sample ID from one process to another until the process has completed all processes within the recipes assigned to it. Here is an example where a sample is first aliquoted, then extracted and normalized and then an assay is performed and then analyzed:
![]()
Figure 1 – Basic Wet Lab Workflow
In this example, all the listed steps are traditionally considered part of the wet lab operations. However, there is much more that goes into processing samples than merely the processes done in the lab. A LIMS that is capable of interfacing with multiple systems outside of the wet lab ensures end-to-end connectivity of data and a more cohesive informatic solution. See Figure 2 for examples of end-to-end connectivity of data. This example could represent a laboratory processing consumer genomic samples where a customer portal is used to register kits; an integrated ERP system manages inventory; connectivity with a commercial shipping system updates tracked kits and samples received automatically; and wet lab operations are processing samples, requesting inventory when needed, quarantining reagents until reagents pass QC and analyzing samples.
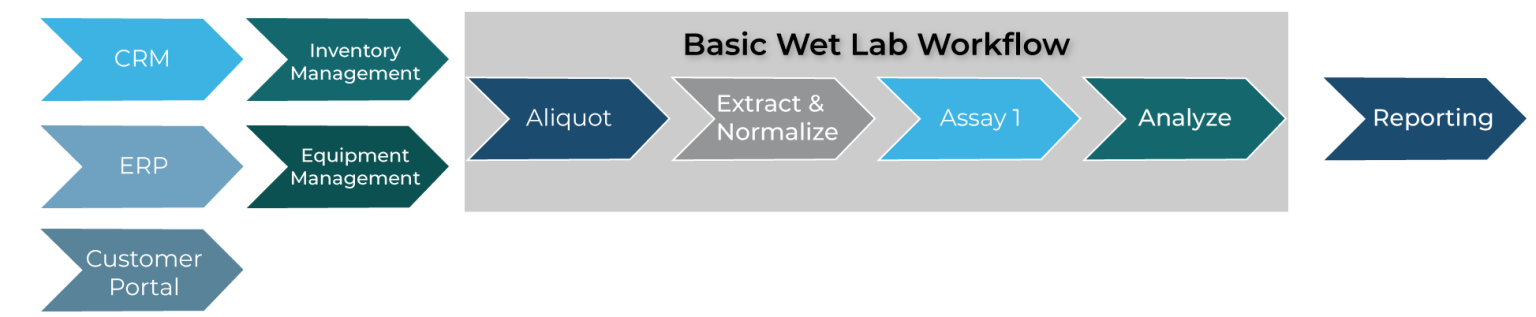
Figure 2 – End-to-End Workflow Example with Integrations to Multiple Systems
An end-to-end LIMS solution requires thorough understanding of all aspects of a lab’s operations. Too often a company who decides to invest in a LIMS starts with several internal meetings gathering requirements of a LIMS solution. However, the company that prepares a list of 300+ LIMS requirements and then sends that list to multiple LIMS companies asking for demos and bids often fails in executing on the overall project. Why? The reason is simple. The customer is not a LIMS developer and doesn’t understand the requirements from a LIMS perspective. Having developed LIMS solutions for clinical, research and manufacturing environments around the world, BioSoft Integrators takes a fresh approach to tailoring a LIMS for a customer’s needs. With 20+ years’ experience in developing and integrating LIMS systems, we ask clients about the lab operations they need to run by diving into the details about what data is being transferred; what instruments are being integrated; what metadata is being tracked and what processes need to happen in what order to ensure results are correct? And when we understand the current processes, we ask about scale and we ask about efficiencies and growth plans and failures, and, and, and – so the solution we build is prepared for the laboratory’s future needs. We pride ourselves in protecting the investment made by our Clients and we do our best to ensure as much flexibility and scalability in designed into the system from the start, at minimal cost. To revert things back to the traditional 300+ requirements list introduced earlier, we ask our customers at least 300 questions and then we create LIMS requirements aligned with the specific operations of the lab’s SOPs and processes. Most LIMS vendors look at implementation of a LIMS from the 30,000-foot level. “Oh, you need a PCR workflow; we have a canned workflow for that”; we conversely dig-in and get into the weeds and understand the need for a PCR workflow and all the intricacies to ensure ease of use and rapid adoption. Our customers tell us they have never been interrogated about the finer details of their operations like they have by us, and our response is that, the “Devil is in the Details”. It’s far better to understand the requirements in the beginning, than to learn of the gaps in those requirements once deployment is underway.
LIMS solutions can be quite basic, and some can be very comprehensive and interface with all facets of the company, but no matter what phase your business is in, trusting your informatic needs to experts is sound advice. When Excel just isn’t cutting it, give us a call and we’ll tailor a solution to address your lab and your needs. A LIMS quite simply, can (and likely should be) the glue that holds your lab together.
BioSoft Integrators
Founded in 2012, BioSoft Integrators offers professional services and products specializing in informatics. BioSoft’s High Performance Computing (HPC) on premise solutions are turnkey appliances designed for the application of NGS which enable genomic labs to start analyzing data within 4 hours of installation. BioSoft’s LabOptimize™ LIMS informatically ties the whole lab together in an affordable and scalable way. For general development and testing needs, BioSoft’s software development and testing professionals have expertise with scientific experience. The company’s motto is Your Lab, Your Way. More information can be found on their website at www.biosoftintegrators.com.

